Papers
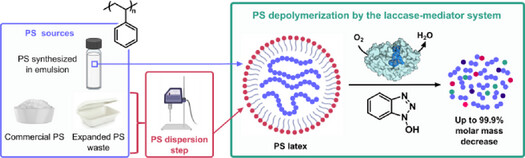
14. Harnessing Colloidal Dispersion for Laccase-Driven Enzymatic Depolymerization of Polystyrene
M. Pujol, S. A. Gonsales, F. Seksek, J.-G. Berrin, B. Bissaro, D. Taton
Angew. Chem. Int. Ed. 2025, e13937
https://doi.org/10.1002/anie.202513937
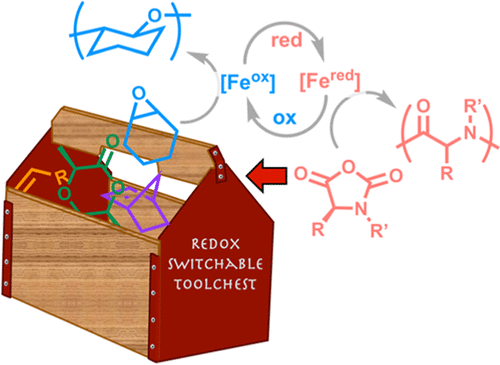
13. Adding Polypeptides to the Toolbox for Redox-Switchable Polymerization and Copolymerization Catalysis
M. S. Thompson, S. A. Johnson, S. A. Gonsales, G. M. Brown, S. L. Kristufek, J. A. Byers
Macromolecules 2023, 56, 3024–3035 https://doi.org/10.1021/acs.macromol.2c02381
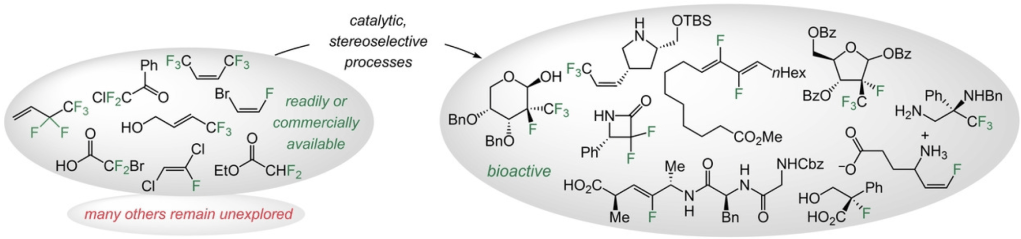
12. Catalytic and Stereoselective Transformations with Easily Accessible and Purchasable Allyl and Alkenyl Fluorides
P. H. S. Paioti,* S. A. Gonsales,* S. Xu, A. Nikbakht, D. C. Fager, Q. Liu, A. H. Hoveyda.*
Angew. Chem. Int. Ed. 2022, e202208742
https://doi.org/10.1002/ange.202208742
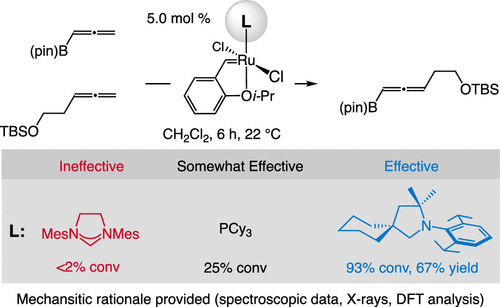
11. Cross-metathesis of Allenes. Mechanistic Analysis and Identification of a Ru-CAAC as the Most Effective Catalyst
S. A. Gonsales, Z. C. Mueller, F. Zhao, P. H. S. Paioti, L. Karmazin, J. Wan, F. Liu, K. N. Houk, A. H. Hoveyda
J. Am. Chem. Soc. 2021, 143, 20640–20644
https://doi.org/10.1021/jacs.1c11453
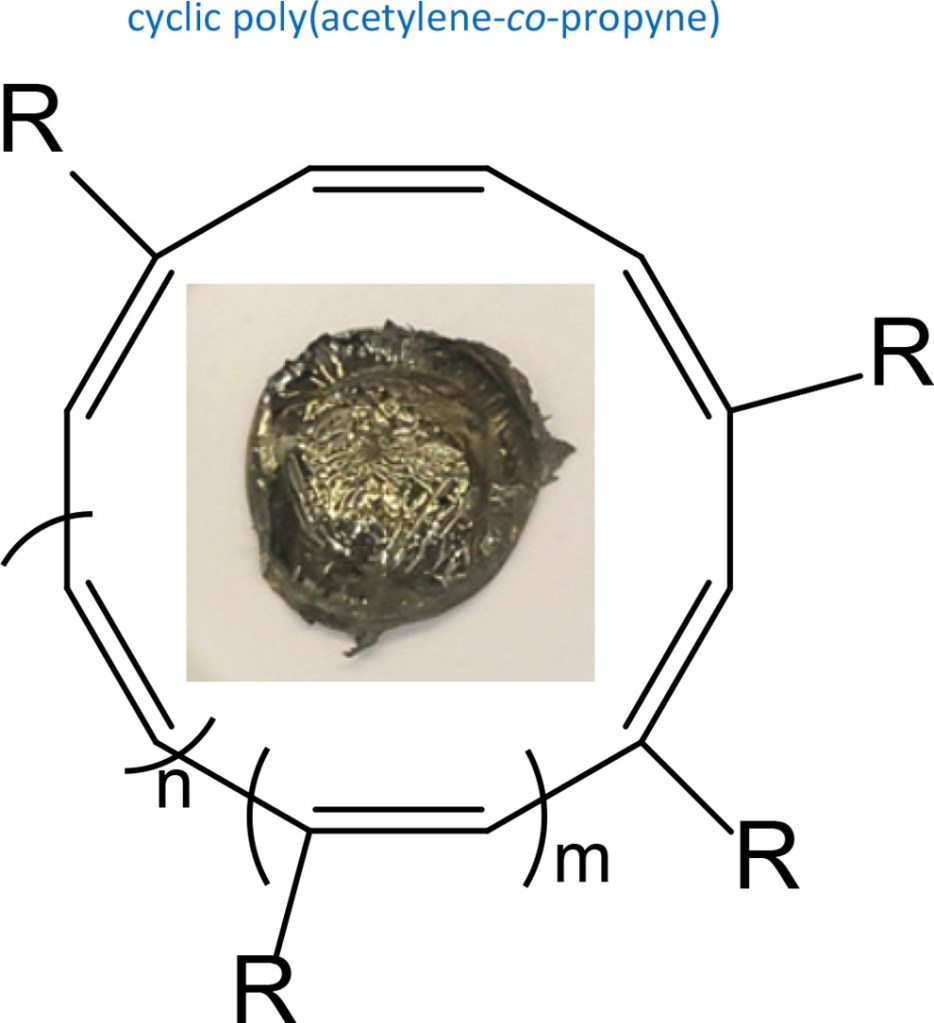
10. Semi-conducting cyclic copolymers of acetylene and propyne
Z. Miao, A. M. Esper, S. S. Nadif, S. A. Gonsales, B. S. Sumerlin, A. S. Veige
React. Funct. Polym. 2021, 169, 105088
https://doi.org/10.1016/j.reactfunctpolym.2021.105088
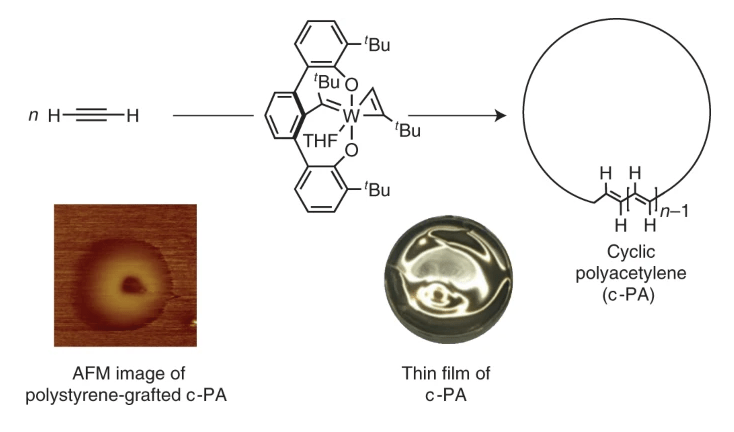
9. Cyclic polyacetylene
Z. Miao, S. A. Gonsales, C. Ehm, F. Mentink-Vigier, C. R. Bowers, B. S. Sumerlin, A. S. Veige
Nature Chem. 2021, 13, 792–799
https://doi.org/10.1038/s41557-021-00713-2
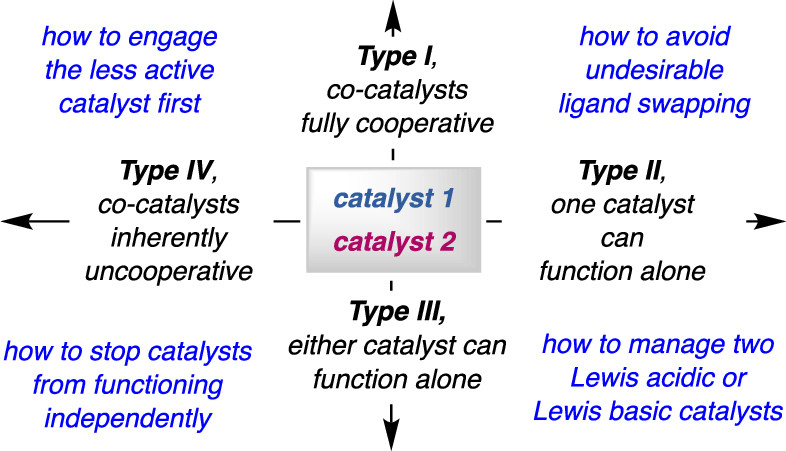
8. Different strategies for designing dual-catalytic enantioselective processes: from fully cooperative to non-cooperative systems
F. Romiti, J. Del Pozo, P. H. S. Paioti, S. A. Gonsales, X. Li, F. W. W. Hartrampf, A. H. Hoveyda
J. Am. Chem. Soc. 2019, 141, 17952–17961
https://doi.org/10.1021/jacs.9b05464
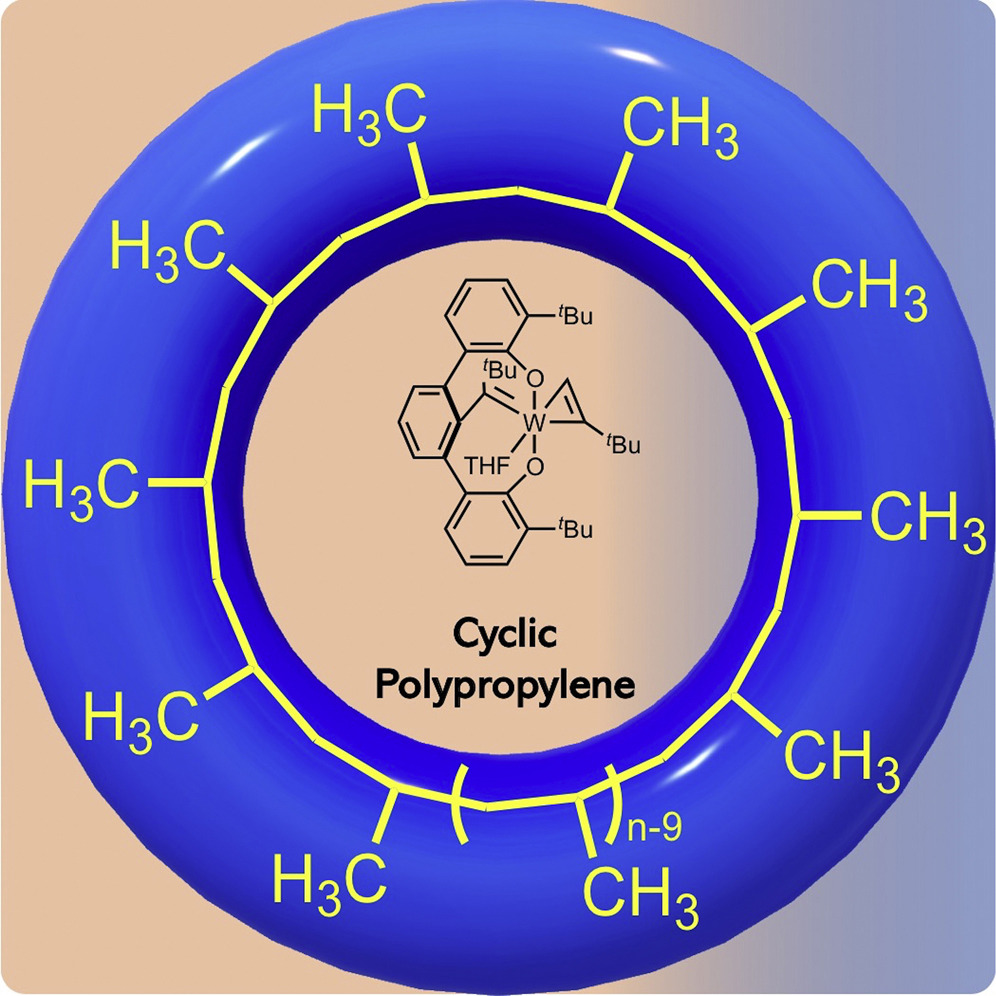
7. Polypropylene: now available without chain ends
W. Niu, S. A. Gonsales, T. Kubo, K. C. Bentz, D. Pal, D. A. Savin, B. S. Sumerlin, A. S. Veige
Chem 2019, 5, 237–244
https://doi.org/10.1016/j.chempr.2018.12.005
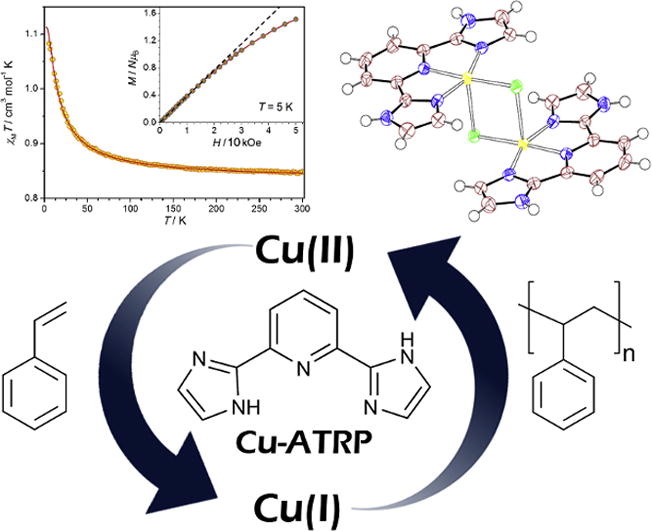
6. Synthesis, structural and magnetic characterization of a copper (II) complex of 2, 6-di (1H-imidazol-2-yl) pyridine and its application in copper-mediated polymerization catalysis
E. G. R. de Arruda, M. A. de Farias, S. A. V. Jannuzzi, S. A. Gonsales, R. A. Timm, S. Sharma, G. Zoppellaro, L. T. Kubota, M. Knobel, A. L. B. Formiga
Inorg. Chim. Acta 2017, 466, 456–463
https://doi.org/10.1016/j.ica.2017.06.073
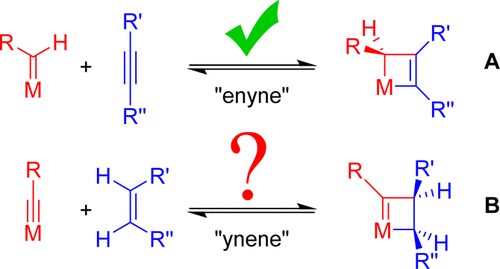
5. Introducing “ynene” metathesis: ring-expansion metathesis polymerization leads to highly cis and syndiotactic cyclic polymers of norbornene
S. S. Nadif, T. Kubo, S. A. Gonsales, S. VenkatRamani, I. Ghiviriga, B. S. Sumerlin, A. S. Veige
J. Am. Chem. Soc. 2016, 138, 6408–6411
https://doi.org/10.1021/jacs.6b03247
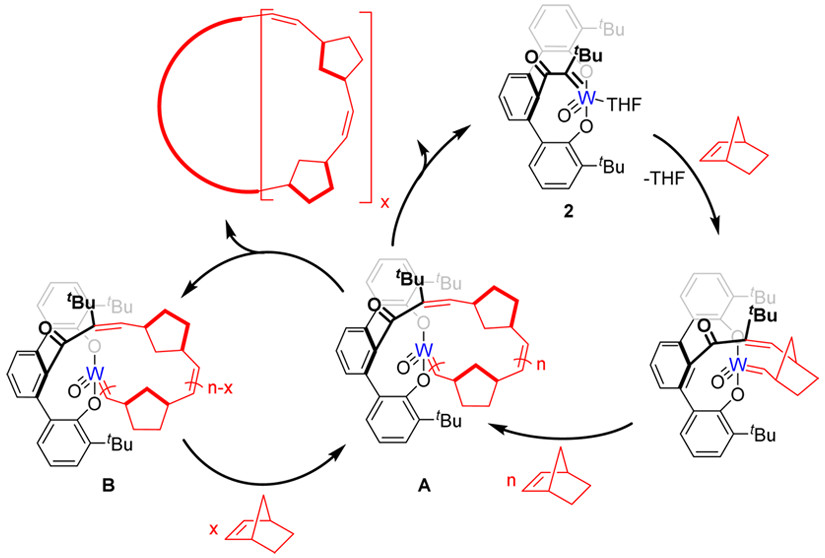
4. Highly tactic cyclic polynorbornene: Stereoselective ring expansion metathesis polymerization of norbornene catalyzed by a new tethered tungsten-alkylidene catalyst
S. A. Gonsales, T. Kubo, M. K. Flint, K. A. Abboud, B. S. Sumerlin, A. S. Veige
J. Am. Chem. Soc. 2016, 138, 4996–4999
https://doi.org/10.1021/jacs.6b00014
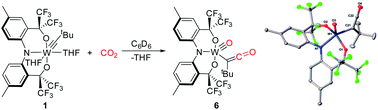
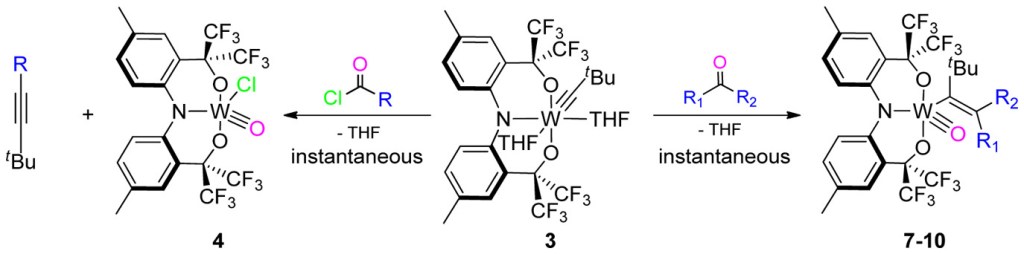
3. Carbon dioxide cleavage across a tungsten-alkylidyne bearing a trianionic pincer-type ligand
S. A. Gonsales, I. Ghiviriga, K. A. Abboud, A. S. Veige
Dalton Trans. 2016, 45, 15783–15785
10.1039/C6DT01049K
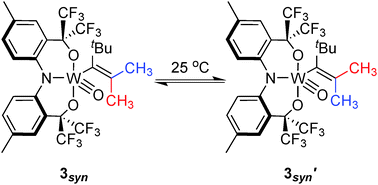
2. Evidence for a zwitterionic transition state in double bond rotations within tungsten–vinyl complexes
S. A. Gonsales, M. E. Pascualini, I. Ghiviriga, A. S. Veige
Chem Comm. 2015, 51, 13404–13407
10.1039/C5CC04851F
1. Fast “wittig-like” reactions as a consequence of the inorganic enamine effect
S. A. Gonsales, M. E. Pascualini, I. Ghiviriga, K. A. Abboud, A. S. Veige
J. Am. Chem. Soc. 2015, 137, 4840–4845
https://doi.org/10.1021/jacs.5b01599
PaTENTS
2. Cyclic polyacetylene and methods of preparing the same
A. S. Veige, S. A. Gonsales, Z. Miao, B. S. Sumerlin.
US Patent App. 17/797,770, 2023 https://patents.google.com/patent/US20230096942A1/en
1. Catalyst for ring expansion metathesis polymerization of cyclic monomers
A. S. Veige, S. A. Gonsales.
US Patent 11,241,681, 2022
https://patents.google.com/patent/US11241681B2/en